Ashima Sharda Mahindra • 06 Feb 2025
FDA Approves Clinical Trials for Pig Kidney Transplants in Humans
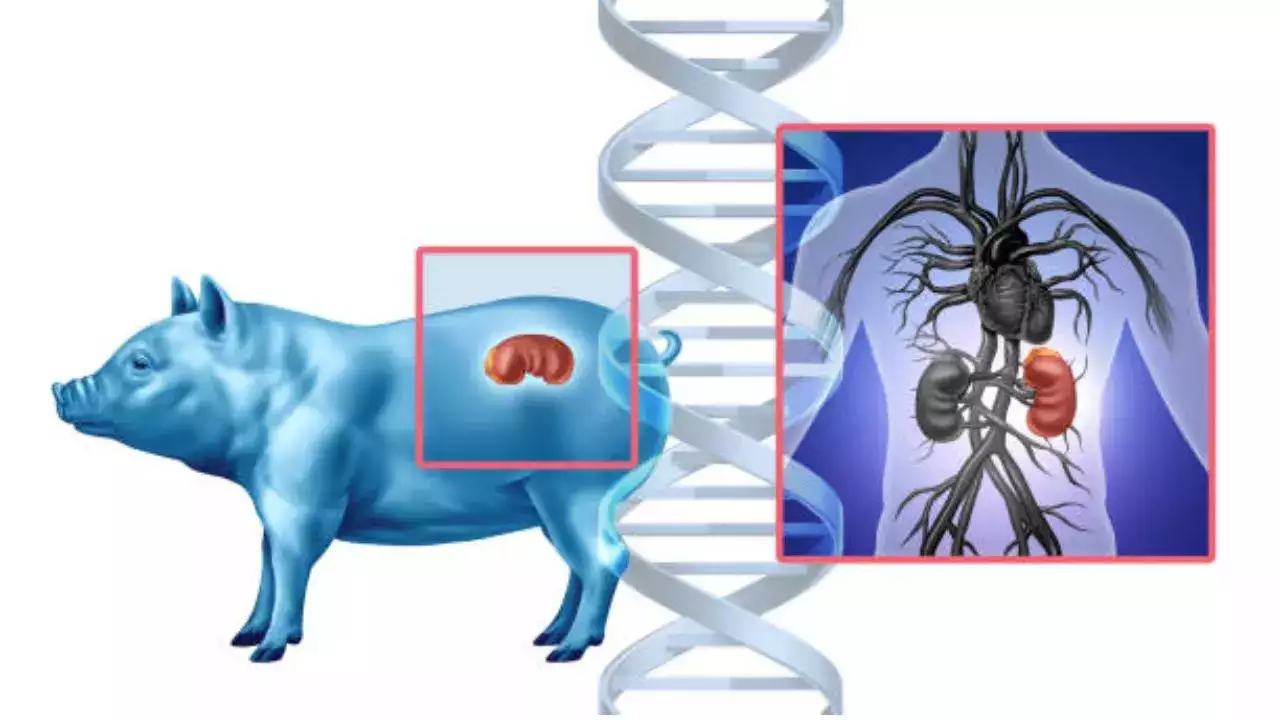
According to the biotechnology companies, the trials are expected to begin by mid-2025
In a major step towards cross-species transplantation, the US Food and Drug Administration has approved the first-ever clinical trials for testing pig kidney transplants in those who have kidney failure. Two biotechnology companies—United Therapeutics Corporation and eGenesis—have been given clearance to begin the research.
If they are successful, the trials could pave the way for new ways to transplant organs in a bid to address a severe shortage of donor kidneys. “We are entering a transformative era in organ transplantation,” The New York Times quoted Mike Curtis, president and chief executive of eGenesis.
According to United Therapeutics Corporation, they will begin the study with six patients, all of whom have been on dialysis for at least six months and have no other serious medical issues. The trial will then expand to 50 partici depending on how the initial studies go. eGenesis said they will start with three patients, increasing the number in later stages.
The Times said all the patients will be monitored for 24 weeks and will need lifelong follow-ups to track health outcomes and potential risks like bacterial and viral infections that can easily cross from pigs to humans.
Pig kidneys are the best solution for human kidney transplant shortage
According to statistics, more than 550,000 Americans have kidney failure, and nearly 100,000 people are on the transplant waiting list. Despite this number, less than 25,000 kidney transplants are performed every year, and many patients wait years or die before receiving a donor organ.
Genetically engineered pigs offer a promising solution because their organs are similar in size and function to human kidneys. Both of the companies have modified pig genes to reduce the risk of organ rejection and improve compatibility with human bodies.
United Therapeutics pigs have undergone 10 gene edits, adding six human genes and removing four porcine genes linked to rejection. Similarly, eGenesis pigs have undergone 69 gene edits, most to inactivate viruses that could pose a risk to humans.
Safety and ethics concerns are there
While there are many potential benefits of cross-species transplantation, experts are also concerned about safety and ethics. Scientists say infections can be carried from pigs to humans—many of them life-threatening.
Also, many are worried that patients who are on the waiting list for organ transplants may not be in the position to truly give consent, considering how difficult it is for those on dialysis to refuse a potentially life-saving solution. And so, fully understanding the lifelong implications of the decision can be hard to live with.
Trials to begin by mid-2025
According to both companies, trial transplants are expected to begin by mid-2025, with a few months between procedures to assess each outcome before moving forward. eGenesis plans to wait six months between its first two patients, then three months before transplanting a third.
The Times says even if the trials are positive and successful, it is not known what the cost of pig organ transplants will be and whether insurance will cover them.
Get Latest News Live on Times Now along with Breaking News and Top Headlines from Health and around the world.